C reative
Particle
Higgs
CPH Theory is based on Generalized light velocity from energy into mass.
CPH Theory in Journals
|
Is Quantum mechanics controlling your thought?
|
|
Science's weirdest realm may be responsible for photosynthesis, our sense of smell, and even consciousness itself.
A sea slug neuron may tap quantum forces to process information.
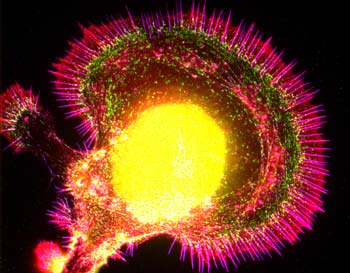
In Graham Fleming sits down at an L-shaped lab bench, occupying a footprint about the size of two parking spaces. Alongside him, a couple of off-the-shelf lasers spit out pulses of light just millionths of a billionth of a second long. After snaking through a jagged path of mirrors and lenses, these minuscule flashes disappear into a smoky black box containing proteins from green sulfur bacteria, which ordinarily obtain their energy and nourishment from the sun. Inside the black box, optics manufactured to billionths-of-a-meter precision detect something extraordinary: Within the bacterial proteins, dancing electrons make seemingly impossible leaps and appear to inhabit multiple places at once. Peering deep into these proteins, Fleming and his colleagues at the University of California at Berkeley and at Washington University in St. Louis have discovered the driving engine of a key step in photosynthesis, the process by which plants and some microorganisms convert water, carbon dioxide, and sunlight into oxygen and carbohydrates. More efficient by far in its ability to convert energy than any operation devised by man, this cascade helps drive almost all life on earth. Remarkably, photosynthesis appears to derive its ferocious efficiency not from the familiar physical laws that govern the visible world but from the seemingly exotic rules of quantum mechanics, the physics of the subatomic world. Somehow, in every green plant or photosynthetic bacterium, the two disparate realms of physics not only meet but mesh harmoniously. Welcome to the strange new world of quantum biology. On the face of things, quantum mechanics and the biological sciences do not mix. Biology focuses on larger-scale processes, from molecular interactions between proteins and DNA up to the behavior of organisms as a whole; quantum mechanics describes the often-strange nature of electrons, protons, muons, and quarks—the smallest of the small. Many events in biology are considered straightforward, with one reaction begetting another in a linear, predictable way. By contrast, quantum mechanics is fuzzy because when the world is observed at the subatomic scale, it is apparent that particles are also waves: A dancing electron is both a tangible nugget and an oscillation of energy. (Larger objects also exist in particle and wave form, but the effect is not noticeable in the macroscopic world.) Quantum mechanics holds that any given particle has a chance of being in a whole range of locations and, in a sense, occupies all those places at once. Physicists describe quantum reality in an equation they call the wave function, which reflects all the potential ways a system can evolve. Until a scientist measures the system, a particle exists in its multitude of locations. But at the time of measurement, the particle has to “choose” just a single spot. At that point, quantum physicists say, probability narrows to a single outcome and the wave function “collapses,” sending ripples of certainty through space-time. Imposing certainty on one particle could alter the characteristics of any others it has been connected with, even if those particles are now light-years away. (This process of influence at a distance is what physicists callentanglement.) As in a game of dominoes, alteration of one particle affects the next one, and so on.
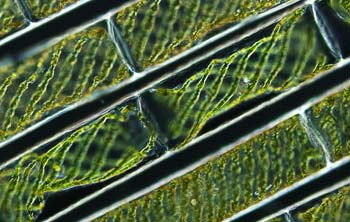
Green algae may rely on quantum computing to turn sunlight into
food. The implications of all this are mind-bending. In the macro world, a ball never spontaneously shoots itself over a wall. In the quantum world, though, an electron in one biomolecule might hop to a second biomolecule, even though classical laws of physics hold that the electrons are too tightly bound to leave. The phenomenon of hopping across seemingly forbidden gaps is called quantum tunneling. From tunneling to entanglement, the special properties of the quantum realm allow events to unfold at speeds and efficiencies that would be unachievable with classical physics alone. Could quantum mechanisms be driving some of the most elegant and inexplicable processes of life? For years experts doubted it: Quantum phenomena typically reveal themselves only in lab settings, in vacuum chambers chilled to near absolute zero. Biological systems are warm and wet. Most researchers thought the thermal noise of life would drown out any quantum weirdness that might rear its head. Yet new experiments keep finding quantum processes at play in biological systems, says Christopher Altman, a researcher at the Kavli Institute of Nanoscience in the Netherlands. With the advent of powerful new tools like femtosecond (10-15 second) lasers and nanoscale-precision positioning, life’s quantum dance is finally coming into view.
INTO THE LIGHT The secret, Fleming and his colleagues found, is quantum physics. To unearth the bacteria’s inner workings, the researchers zapped the connective proteins with multiple ultrafast laser pulses. Over a span of femtoseconds, they followed the light energy through the scaffolding to the cellular reaction centers where energy conversion takes place. Then came the revelation: Instead of haphazardly moving from one connective channel to the next, as might be seen in classical physics, energy traveled in several directions at the same time. The researchers theorized that only when the energy had reached the end of the series of connections could an efficient pathway retroactively be found. At that point, the quantum process collapsed, and the electrons’ energy followed that single, most effective path. Electrons moving through a leaf or a green sulfur bacterial bloom are effectively performing a quantum “random walk”—a sort of primitive quantum computation—to seek out the optimum transmission route for the solar energy they carry. “We have shown that this quantum random-walk stuff really exists,” Fleming says. “Have we absolutely demonstrated that it improves the efficiency? Not yet. But that’s our conjecture. And a lot of people agree with it.”
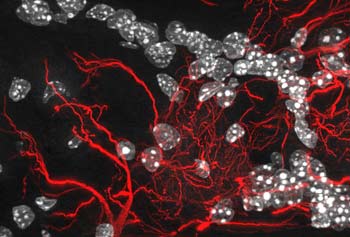
The olfactory bulb of an adult mouse (seen here at 800x
magnification) Elated by the finding, researchers are looking to mimic nature’s quantum ability to build solar energy collectors that work with near-photosynthetic efficiency. Alán Aspuru-Guzik, an assistant professor of chemistry and chemical biology at Harvard University, heads a team that is researching ways to incorporate the quantum lessons of photosynthesis into organic photovoltaic solar cells. This research is in only the earliest stages, but Aspuru-Guzik believes that Fleming’s work will be applicable in the race to manufacture cheap, efficient solar power cells out of organic molecules.
TUNNELING FOR SMELL What is really happening, Turin posited, is that the approximately 350 types of human smell receptors perform an act of quantum tunneling when a new odorant enters the nostril and reaches the olfactory nerve. After the odorant attaches to one of the nerve’s receptors, electrons from that receptor tunnel through the odorant, jiggling it back and forth. In this view, the odorant’s unique pattern of vibration is what makes a rose smell rosy and a wet dog smell wet-doggy.
In 2007 Turin (who is now chief technical officer of the odorant-designing companyFlexitral in Chantilly, Virginia) and his hypothesis received support from a paper by four physicists at University College London. That work, published in the journalPhysical Review Letters [subscription required], showed how the smell-tunneling process may operate. As an odorant approaches, electrons released from one side of a receptor quantum-mechanically tunnel through the odorant to the opposite side of the receptor. Exposed to this electric current, the heavier pinanethiol would vibrate differently from the lighter but similarly shaped pinanol. “I call it the ‘swipe-card model,’?” says coauthor A. Marshall Stoneham, an emeritus professor of physics. “The card’s got to be a good enough shape to swipe through one of the receptors.” But it is the frequency of vibration, not the shape, that determines the scent of a molecule.
THE GREEN TEA PARTY Free radical molecules, by-products of the body’s breakdown of food or environmental toxins, have a spare electron. That extra electron makes free radicals reactive, and hence dangerous as they travel through the bloodstream. But an electron from the catechin can make use of quantum mechanics to tunnel across the gap to the free radical. Suddenly the catechin has chemically bound up the free radical, preventing it from interacting with and damaging cells in the body. Quantum tunneling has also been observed in enzymes, the proteins that facilitate molecular reactions within cells. Two studies, one published in Science in 2006 and the other in Biophysical Journal in 2007, have found that some enzymes appear to lack the energy to complete the reactions they ultimately propel; the enzyme’s success, it now seems, could be explained only through quantum means.
QUANTUM TO THE CORE Hameroff speculates that anesthetics “interrupt a delicate quantum process” within the neurons of the brain. Each neuron contains hundreds of long, cylindrical protein structures, called microtubules, that serve as scaffolding. Anesthetics, Hameroff says, dissolve inside tiny oily regions of the microtubules, affecting how some electrons inside these regions behave. He speculates that the action unfolds like this: When certain key electrons are in one “place,” call it to the “left,” part of the microtubule is squashed; when the electrons fall to the “right,” the section is elongated. But the laws of quantum mechanics allow for electrons to be both “left” and “right” at the same time, and thus for the microtubules to be both elongated and squashed at once. Each section of the constantly shifting system has an impact on other sections, potentially via quantum entanglement, leading to a dynamic quantum-mechanical dance. It is in this faster-than-light subatomic communication, Hameroff says, that consciousness is born. Anesthetics get in the way of the dancing electrons and stop the gyration at its quantum-mechanical core; that is how they are able to switch consciousness off. It is still a long way from Hameroff’s hypothetical (and experimentally unproven) quantum neurons to a sentient, conscious human brain. But many human experiences, Hameroff says, from dreams to subconscious emotions to fuzzy memory, seem closer to the Alice in Wonderland rules governing the quantum world than to the cut-and-dried reality that classical physics suggests. Discovering a quantum portal within every neuron in your head might be the ultimate trip through the looking glass. Source: http://discovermagazine.com/2009/feb/13-is-quantum-mechanics-controlling-your-thoughts
1 2 3 4 5 6 7 8 9 10 Newest articles
|
|
Sub quantum space and interactions from photon to fermions and bosons |
Interesting articles
Since 1962 I doubted on Newton's laws. I did not accept the infinitive speed and I found un-vivid the laws of gravity and time.
I learned the Einstein's Relativity, thus I found some answers for my questions. But, I had another doubt of Infinitive Mass-Energy. And I wanted to know why light has stable speed?