C reative
Particle
Higgs
CPH Theory is based on Generalized light velocity from energy into mass.
CPH Theory in Journals
|
The Hydrogen Economy
|
|
The Hydrogen Economy
If the fuel cell is to become the modern steam engine, basic research must provide breakthroughs in understanding, materials, and design to make a hydrogen−based energy system a vibrant and competitive force.
Since the industrial revolution began in the 18th century, fossil fuels in the form of coal, oil, and natural gas have powered the technology and transportation networks that drive society. But continuing to power the world from fossil fuels threatens our energy supply and puts enormous strains on the environment. The world's demand for energy is projected to double by 2050 in response to population growth and the industrialization of developing countries.1 The supply of fossil fuels is limited, with restrictive shortages of oil and gas projected to occur within our lifetimes (see the article by Paul Weisz in Physics Today, July 2004, page 47). Global oil and gas reserves are concentrated in a few regions of the world, while demand is growing everywhere; as a result, a secure supply is increasingly difficult to assure. Moreover, the use of fossil fuels puts our own health at risk through the chemical and particulate pollution it creates. Carbon dioxide and other greenhouse gas emissions that are associated with global warming threaten the stability of Earth's climate. A replacement for fossil fuels will not appear overnight. Extensive R&D is required before alternative sources can supply energy in quantities and at costs competitive with fossil fuels, and making those alternative sources available commercially will itself require developing the proper economic infrastructure. Each of those steps takes time, but greater global investment in R&D will most likely hasten the pace of economic change. Although it is impossible to predict when the fossil fuel supply will fall short of demand or when global warming will become acute, the present trend of yearly increases in fossil fuel use shortens our window of opportunity for a managed transition to alternative energy sources. Hydrogen as energy carrierOne promising alternative to fossil fuels is hydrogen2,3 (see the article by Joan Ogden, Physics Today, April 2002, page 69). Through its reaction with oxygen, hydrogen releases energy explosively in heat engines or quietly in fuel cells to produce water as its only byproduct. Hydrogen is abundant and generously distributed throughout the world without regard for national boundaries; using it to create a hydrogen economy—a future energy system based on hydrogen and electricity—only requires technology, not political access. Although in many ways hydrogen is an attractive replacement for fossil fuels, it does not occur in nature as the fuel H2. Rather, it occurs in chemical compounds like water or hydrocarbons that must be chemically transformed to yield H2. Hydrogen, like electricity, is a carrier of energy, and like electricity, it must be produced from a natural resource. At present, most of the world's hydrogen is produced from natural gas by a process called steam reforming. However, producing hydrogen from fossil fuels would rob the hydrogen economy of much of its raison d'être: Steam reforming does not reduce the use of fossil fuels but rather shifts them from end use to an earlier production step; and it still releases carbon to the environment in the form of CO2. Thus, to achieve the benefits of the hydrogen economy, we must ultimately produce hydrogen from non−fossil resources, such as water, using a renewable energy source.
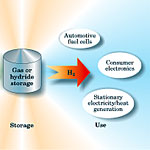
Figure 1 depicts the hydrogen economy as a network composed of three functional steps: production, storage, and use. There are basic technical means to achieve each of these steps, but none of them can yet compete with fossil fuels in cost, performance, or reliability. Even when using the cheapest production method—steam reforming of methane—hydrogen is still four times the cost of gasoline for the equivalent amount of energy. And production from methane does not reduce fossil fuel use or CO2 emission. Hydrogen can be stored in pressurized gas containers or as a liquid in cryogenic containers, but not in densities that would allow for practical applications—driving a car up to 500 kilometers on a single tank, for example. Hydrogen can be converted to electricity in fuel cells, but the production cost of prototype fuel cells remains high: $3000 per kilowatt of power produced for prototype fuel cells (mass production could reduce this cost by a factor of 10 or more), compared with $30 per kilowatt for gasoline engines. The gap between the present state of the art in hydrogen production, storage, and use and that needed for a competitive hydrogen economy is too wide to bridge in incremental advances. It will take fundamental breakthroughs of the kind that come only from basic research. Beyond reformingThe US Department of Energy estimates that by 2040 cars and light trucks powered by fuel cells will require about 150 megatons per year of hydrogen.3 The US currently produces about 9 megatons per year, almost all of it by reforming natural gas. The challenge is to find inexpensive and efficient routes to create hydrogen in sufficient quantities from non−fossil natural resources. The most promising route is splitting water, which is a natural carrier of hydrogen. It takes energy to split the water molecule and release hydrogen, but that energy is later recovered during oxidation to produce water. To eliminate fossil fuels from this cycle, the energy to split water must come from non−carbon sources, such as the electron−hole pairs excited in a semiconductor by solar radiation, the heat from a nuclear reactor or solar collector, or an electric voltage generated by renewable sources such as hydropower or wind. The direct solar conversion of sunlight to H2 is one of the most fascinating developments in water splitting.4 Established technology splits water in two steps: conversion of solar radiation to electricity in photovoltaic cells followed by electrolysis of water in a separate cell. It is well known that the photovoltaic conversion occurs with an efficiency up to 32% when expensive single−crystal semiconductors are used in multi−junction stacks, or about 3% with much cheaper organic semiconductors; remarkably, the cost of delivered electricity is about the same in both cases. Advanced electrolyzers split water with 80% efficiency. The two processes, however, can be combined in a single nanoscale process: Photon absorption creates a local electron−hole pair that electrochemically splits a neighboring water molecule. The efficiency of this integrated photochemical process can be much higher, in principle, than the two sequential processes; it has now reached 8−12% in the laboratory4 and has prospects for much greater gains as researchers learn to better control the nanoscale excitation and photochemistry. The technical challenge is finding robust semiconductor materials that satisfy the competing requirements of nature. The Sun's photons are primarily in the visible, a wavelength that requires semiconductors with small bandgaps—below 1.7 eV—for efficient absorption. Oxide semiconductors like titanium dioxide that are robust in aqueous environments have wide bandgaps, as high as 3.0 eV, and thus require higher−energy photons for excitation. The use of dye−sensitized photocells that accumulate energy from multiple low−energy photons to inject higher−energy electrons into the semiconductor is a promising direction for matching the solar spectrum. Alternatively, oxide semiconductors can be doped with impurities that reduce their bandgap energies to overlap better with the solar spectrum. In both cases, new strategies for nanostructured hybrid materials are needed to more efficiently use solar energy to split water. Water can be split in thermochemical cycles operating at elevated temperatures to facilitate the reaction kinetics.5 Heat sources include solar collectors operating up to 3000°C or nuclear reactors designed to operate between 500°C and 900°C (see the article by Gail Marcus and Alan Levin, Physics Today, April 2002, page 54). More than 100 types of chemical cycles have been proposed, including systems based on zinc−oxygen operating at 1500°C, sulfur−iodine at 850°C, calcium−bromine at 750°C, and copper−chlorine at 550°C. At high temperatures, thermochemical cycles must deal with the tradeoff between favorable reaction kinetics and aggressive chemical corrosion of containment vessels. Separating the reaction products at high temperature is a second challenge: Unseparated mixtures of gases recombine if allowed to cool. But identifying effective membrane materials that selectively pass hydrogen, oxygen, water, hydrogen sulfate, or hydrogen iodide, for example, at high temperature remains a problem. Dramatic improvements in catalysis could lower the operating temperature of thermochemical cycles, and thus reduce the need for high−temperature materials, without losing efficiency. Molecular−level challenges, with which researchers are fast making progress using nanoscale design, include accelerating the kinetics of reactions through catalysis, separating the products at high temperature, and directing products to the next reaction step.
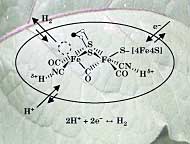
Bio−inspired processes offer stunning opportunities to approach the hydrogen production problem anew.6 The natural world began forming its own hydrogen economy 3 billion years ago, when it developed photosynthesis to convert CO2, water, and sunlight into hydrogen and oxygen. Plants use hydrogen to manufacture the carbohydrates in their leaves and stalks, and emit oxygen to the atmosphere for animals to breathe. Single−cell organisms such as algae and many microbes produce hydrogen efficiently at ambient temperatures by molecular−level processes. These natural mechanisms for producing hydrogen involve elaborate protein structures that have only recently been partially solved. For billions of years, for instance, plants have used a catalyst based on manganese−oxygen clusters to split water efficiently at room temperature, a process that frees protons and electrons. Likewise, bacteria use iron and nickel clusters as the active elements both for combining protons and electrons into H2 and splitting H2 into protons and electrons (see Figure 2). The hope is that researchers can capitalize on nature's efficient manufacturing processes by fully understanding molecular structures and functions and then imitating them using artificial materials in such applications as fuel−cell anodes and cathodes. Storing hydrogenStoring hydrogen in a high−energy−density form that flexibly links its production and eventual use is a key element of the hydrogen economy. Unlike electricity, which must be produced and used at the same rate, stored hydrogen can be stockpiled for much later use, or used as ballast to bridge the differing temporal cycles of energy production and consumption. The traditional storage options are conceptually simple—cylinders of liquid and high−pressure gas. Industrial facilities and laboratories are already accustomed to handling hydrogen both ways. These options are viable for the stationary consumption of hydrogen in large plants that can accommodate large weights and volumes. Storage as liquid H2 imposes severe energy costs because up to 40% of its energy content can be lost to liquefaction
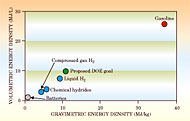
For transportation use, the on−board storage of hydrogen is a far more difficult challenge. Both weight and volume are at a premium, and sufficient fuel must be stored to make it practical to drive distances comparable to gas−powered cars.3 Figure 3illustrates the challenge by showing the gravimetric and volumetric energy densities of fuels, including the container and apparatus needed for fuel handling. For hydrogen, that added weight is a major fraction of the total. For on−vehicle use, hydrogen need store only about half of the energy that gasoline provides because the efficiency of fuel cells can be greater by a factor of two or more than that of internal combustion engines. Even so, the energy densities of the most advanced batteries and of liquid and gaseous hydrogen pale in comparison to gasoline.
Meeting the volume restrictions in cars or trucks, for instance, requires using hydrogen stored at densities higher than its liquid density. Figure 4 shows the volume density of hydrogen stored in several compounds and in some liquid hydrocarbons.7All of those compounds store hydrogen at higher density than the liquid or the compressed gas at 10 000 psi (Ž700 bar), shown as points on the right−hand vertical axis for comparison. The most effective storage media are located in the upper−right quadrant of the Figure, where hydrogen is combined with light elements like lithium, nitrogen, and carbon. The materials in that part of the plot have the highest mass fraction and volume density of hydrogen. Hydrocarbons like methanol and octane are notable as high−volume−density hydrogen storage compounds as well as high−energy− density fuels, and cycles that allow the fossil fuels to release and recapture their hydrogen are already in use in stationary chemical processing plants.7 The two challenges for on−vehicle hydrogen storage and use are capacity and cycling performance under the accessible on−board conditions of 0−100°C and 1−10 bars. To achieve high storage capacity at low weight requires strong chemical bonds between hydrogen and light−atom host materials in stable compounds, such as lithium borohydride (LiBH4). But to achieve fast cycling at accessible conditions requires weak chemical bonds, fast kinetics, and short diffusion lengths, as might be found in surface adsorption. Thus, the high−capacity and fast−recycling requirements are somewhat in conflict. Many bulk hydrogen−storage compounds, such as metallic magnesium nitrogen hydride (Mg2NH4) and ionic sodium borohydride (Na+(BH4)−), contain high volumetric hydrogen densities but require temperatures of 300°C or more at 1 bar to release their H2. Compounds with low−temperature capture and release behavior, such as lanthanum nickel hydride (LaNi5H6), have low hydrogen−mass fractions and are thus heavy to carry. Hydrogen absorption on surfaces is a potential route to fast cycling, but has been explored relatively little except for carbon substrates. Hydrogen can be adsorbed in molecular or atomic form on suitable surfaces, using pressure, temperature, or electrochemical potential to control its surface structure and bonding strength. A major challenge is controlling the bonding and kinetics of multiple layers of hydrogen. The first layer is bonded by van der Waals or chemical forces specific to the substrate; the second layer sees primarily the first layer and therefore bonds with very different strength. The single−layer properties of adsorbed hydrogen on carbon can be predicted rather accurately and are indicated by the solid curve in Figure 4; the behavior of multiple layers is much less well understood. But experience with carbon suggests that multiple layers are needed for effective storage capacity. One route for overcoming the single−layer limitation is to adsorb hydrogen on both sides of a substrate layer, arranged with others in nanoscale stacks that allow access to both sides. Nanostructured materials offer a host of promising routes for storing hydrogen at high capacity in compounds that have fast recycling. Large surface areas can be coated with catalysts to assist in the dissociation of gaseous H2, and the small volume of individual nanoparticles produces short diffusion paths to the material's interior. The strength of the chemical bonds with hydrogen can be weakened with additives7 such as titanium dioxide in sodium aluminum hydride (NaAlH4). The capture and release cycle is a complex process that involves molecular dissociation, diffusion, chemical bonding, and van der Waals attraction. Each of the steps can be optimized in a specific nanoscale environment that includes appropriate catalysts, defects, and impurity atoms. By integrating the steps into an interactive nanoscale architecture where hydrogen molecules or atoms are treated in one environment for dissociation, for example, and handed off to the next environment for diffusion, nanoscience engineers could simultaneously optimize all the desired properties. Another approach is to use three−dimensional solids with open structures, such as metal−organic frameworks8 in which hydrogen molecules or atoms can be adsorbed on internal surfaces. The metal atoms that form the vertices of such structures can be catalysts or dopants that facilitate the capture and release cycle. Designed nanoscale architectures offer unexplored options for effectively controlling reactivity and bonding to meet the desired storage requirements. Realizing the promiseA major attraction of hydrogen as a fuel is its natural compatibility with fuel cells. The higher efficiency of fuel cells—currently 60% compared to 22% for gasoline or 45% for diesel internal combustion engines—would dramatically improve the efficiency of future energy use. Coupling fuel cells to electric motors, which are more than 90% efficient, converts the chemical energy of hydrogen to mechanical work without heat as an intermediary. This attractive new approach for energy conversion could replace many traditional heat engines. The broad reach of that efficiency advantage is a strong driver for deploying hydrogen fuel cells widely. Although fuel cells are more efficient, there are also good reasons for burning hydrogen in heat engines for transportation. Jet engines and internal combustion engines can be rather easily modified to run on hydrogen instead of hydrocarbons. Internal combustion engines run as much as 25% more efficiently on hydrogen compared to gasoline and produce no carbon emissions. The US and Russia have test−flown commercial airliners with jet engines modified to burn hydrogen.9 Similarly, BMW, Ford, and Mazda are road− testing cars powered by hydrogen internal combustion engines that achieve a range of 300 kilometers, and networks of hydrogen filling stations are being implemented in some areas of the US, Europe, and Japan. Such cars and filling stations could provide an early start and a transitional bridge to hydrogen fuel−cell transportation. The versatility of fuel cells makes them workable in nearly any application where electricity is useful. Stationary plants providing 200 kilowatts of neighborhood electrical power are practical and operating efficiently. Such plants can connect to the electrical grid to share power but are independent of the grid in case of failure. Fuel−cell power for consumer electronics like laptop computers, cell phones, digital cameras, and audio players provide more hours of operation than batteries at the same volume and weight. Although the cost per kilowatt is high for these small units, the unit cost can soon be within an acceptable consumer range. Electronics applications may be the first to widely reach the consumer market, establish public visibility, and advance the learning curve for hydrogen technology. The large homogeneous transportation market offers enormous potential for hydrogen fuel cells to dramatically reduce fossil fuel use, lower harmful emissions, and improve energy efficiency. Fuel cells can be used not only in cars, trucks, and buses, but also can replace the diesel electric generators in locomotives and power all−electric ships.8Europe already has a demonstration fleet of 30 fuel−cell buses running regular routes in 10 cities, and Japan is poised to offer fuel−cell cars for sale
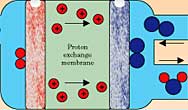
A host of fundamental performance problems remain to be solved before hydrogen in fuel cells can compete with gasoline.10 The heart of the fuel cell is the ionic conducting membrane that transmits protons or oxygen ions between electrodes while electrons go through an external load to do their electrical work, as shown in Figure 5. Each of the half reactions at work in that circuit requires catalysts interacting with electrons, ions, and gases traveling in different media. Designing nanoscale architectures for these triple percolation networks that effectively coordinate the interaction of reactants with nanostructured catalysts is a major opportunity for improving fuel−cell performance. The trick is to get intimate contact of the three phases that coexist in the cell—the incoming hydrogen or incoming oxygen gas phase, an electrolytic proton−conducting phase, and a metallic phase in which electrons flow into or from the external circuit (see Physics Today, July 2001, page 22). A primary factor limiting proton−exchange−membrane (PEM) fuel−cell performance is the slow kinetics of the oxygen reduction reaction at the cathode. Even with the best platinum−based catalysts, the sluggish reaction reduces the voltage output of the fuel cell from the ideal 1.23 V to 0.8 V or less when practical currents are drawn. This voltage reduction is known as the oxygen overpotential. The causes of the slow kinetics, and solutions for speeding up the reaction, are hidden in the complex reaction pathways and intermediate steps of the oxygen reduction reaction. It is now becoming possible to understand this reaction at the atomic level using sophisticated surface−structure and spectroscopy tools such as vibrational spectroscopies, scanning probe microscopy, x−ray diffraction and spectroscopy, and transmission electron microscopy.11,12 In situ electrochemical probes, operating under reaction or near reaction conditions, reveal the energetics, kinetics, and intermediates of the reaction pathway and their relation to the surface structure and composition of the reactants and catalysts. These powerful new experimental probes, combined with equally powerful and impressive computational quantum chemistry using density functional theory,13 are opening a new chapter in atomic−level understanding of the catalytic process. The role of such key features as the atomic con_1figuration of catalysts and their supports, and the electronic structure of surface−reconstructed atoms and adsorbed intermediate species, is within reach of fundamental understanding. These emerging and incisive experimental and theoretical tools make the field of nanoscale electrocatalysis ripe for rapid and comprehensive growth. The research is highly interdisciplinary, incorporating forefront elements of chemistry, physics, and materials science. Beyond the oxygen reduction reaction, fuel cells provide many other challenges. The dominant membrane for PEM fuel cells is perfluorosulfonic acid (PFSA), a polymer built around a C−F backbone with side chains containing sulfonic acid groups (SO3−) (for example, Nafion). Beside its high cost, this membrane must incorporate mobile water molecules into its structure to enable proton conduction. That restricts its operating temperature to below the boiling point of water. At this low temperature—typically around 80°C— expensive catalysts like platinum are required to make the electrochemical reactions sufficiently active, but even trace amounts of carbon monoxide in the hydrogen fuel stream can poison the catalysts. A higher operating temperature would expand the range of suitable catalysts and reduce their susceptibility to poisoning. Promising research directions for alternative proton−conducting membranes that operate at 100−200°C include sulfonating C−H polymers rather than C−F polymers, and using inorganic polymer composites and acid−base polymer blends.14 Solid oxide fuel cells (SOFCs) require O2− transport membranes, which usually consist of perovskite materials containing specially designed defect structures that become sufficiently conductive only above 800°C. The high temperature restricts the construction materials that can be used in SOFCs and limits their use to special environments like stationary power stations or perhaps large refrigerated trucks where adequate thermal insulation and safety can be ensured. Finding new materials that conduct O2− at lower temperatures would significantly expand the range of applications and reduce the cost of SOFCs. OutlookThe hydrogen economy has enormous societal and technical appeal as a potential solution to the fundamental energy concerns of abundant supply and minimal environmental impact. The ultimate success of a hydrogen economy depends on how the market reacts: Does emerging hydrogen technology provide more value than today's fossil fuels? Although the market will ultimately drive the hydrogen economy, government plays a key role in the move from fossil−fuel to hydrogen technology. The investments in R&D are large, the outcome for specific, promising approaches is uncertain, and the payoff is often beyond the market's time horizon. Thus, early government investments in establishing goals, providing research support, and sharing risk are necessary to prime the emergence of a vibrant, market−driven hydrogen economy. The public acceptance of hydrogen depends not only on its practical and commercial appeal, but also on its record of safety in widespread use. The special flammability, buoyancy, and permeability of hydrogen present challenges to its safe use that are different from, but not necessarily more difficult than, those of other energy carriers. Researchers are exploring a variety of issues: hydrodynamics of hydrogen−air mixtures, the combustion of hydrogen in the presence of other gases, and the embrittlement of materials by exposure to hydrogen, for example. Key to public acceptance of hydrogen is the development of safety standards and practices that are widely known and routinely used—like those for self−service gasoline stations or plug−in electrical appliances. The technical and educational components of this aspect of the hydrogen economy need careful attention. Technical progress will come in two forms. Incremental advances of present technology provide low−risk commercial entry into the hydrogen economy. Those advances include improving the yield of natural−gas reforming to lower cost and raise efficiency; improving the strength of container materials for high−pressure storage of hydrogen gas; and tuning the design of internal combustion engines to burn hydrogen. To significantly increase the energy supply and security, and to decrease carbon emission and air pollutants, however, the hydrogen economy must go well beyond incremental advances. Hydrogen must replace fossil fuels through efficient production using solar radiation, thermochemical cycles, or bio−inspired catalysts to split water. Hydrogen must be stored and released in portable solid−state media, and fuel cells that convert hydrogen to electrical power and heat must be put into widespread use. Achieving these technological milestones while satisfying the market discipline of competitive cost, performance, and reliability requires technical breakthroughs that come only from basic research. The interaction of hydrogen with materials encompasses many fundamental questions that can now be explored much more thoroughly than ever before using sophisticated atomic−level scanning probes, in situ structural and spectroscopic tools at x−ray, neutron, and electron scattering facilities, and powerful theory and modeling using teraflop computers. The hope is to solve mysteries that Nature has long kept hidden, such as the molecular basis of catalysis and the mechanism that allows plants to split water at room temperature using sunlight. Nanoscience provides not only new approaches to basic questions about the interaction of hydrogen with materials, but also the power to synthesize materials with custom−designed architectures. This combination of nanoscale analysis and synthesis promises to create new materials technology, such as orderly control of the electronic, ionic, and catalytic processes that regulate the three−phase percolation networks in fuel cells. Such exquisite control over materials behavior has never been so near at hand. The international character of the hydrogen economy is sure to influence how it develops and evolves globally. Each country or region of the world has technological and political interests at stake. Cooperation among nations to leverage resources and create innovative technical and organizational approaches to the hydrogen economy is likely to significantly enhance the effectiveness of any nation that would otherwise act alone. The emphasis of the hydrogen research agenda varies with country; communication and cooperation to share research plans and results are essential. Will the hydrogen economy succeed? Historical precedents suggest that it might. New energy sources and carriers have flourished when coupled with new energy converters. Coal became king as fuel for the steam engine to power the industrial revolution—it transformed the face of land transportation from horse and buggy to rail, and on the sea from sail to steamship.15 Oil fueled the internal combustion engine to provide automobiles and trucks that crisscross continents, and later the jet engine to conquer the skies. Electricity coupled with light bulbs and with rotary motors to power our homes and industries. Hydrogen has its own natural energy−conversion partner, the fuel cell. Together they interface intimately with the broad base of electrical technology already in place, and they can expand to propel cars, locomotives, and ships, power consumer electronics, and generate neighborhood heat and light. Bringing hydrogen and fuel cells to that level of impact is a fascinating challenge and opportunity for basic science, spanning chemistry, physics, biology, and materials. George Crabtree is a physicist in the materials science division at Argonne National Laboratory in Illinois. Mildred Dresselhaus is a professor in the department of physics and the department of electrical engineering and computer science at the Massachusetts Institute of Technology in Cambridge. Michelle Buchanan is a chemist in the chemical sciences division at Oak Ridge National Laboratory in Tennessee. References1. M. I. Hoffert et al., Nature 395, 891 (1998); Energy Information Administration,International Energy Outlook 2004, rep. no. DOE/EIA−0484 (2004), available at http://www.eia. doe.gov/oiaf/ieo. 2. J. A. Turner, Science 285, 687 (1999) [INSPEC]; Special Report: Toward a Hydrogen Economy, Science 305, 957 (2004). 3. US Department of Energy, Office of Basic Energy Sciences, Basic Research Needs for the Hydrogen Economy, US DOE, Washington, DC (2004), available at http://www.sc.doe. gov/bes/hydrogen.pdf; Basic Energy Sciences Advisory Committee, Basic Research Needs to Assure a Secure Energy Future, US DOE, Washington, DC (2003), available at http://www.sc.doe.gov/bes/reports/files/SEF_rpt.pdf; Committee on Alternatives and Strategies for Future Hydrogen Production and Use, The Hydrogen Economy: Opportunities, Costs, Barriers, and R&D Needs, National Research Council, National Academies Press, Washington, DC (2004), available athttp://www.nap.edu/catalog/10922_1.shtml. 4. O. Khasalev, J. A. Turner, Science 280, 425 (1998) [MEDLINE]; S. U. M. Khan, M. Al−Shahry, W. B. Ingler, Science 297, 2189 (2002) [MEDLINE]; N. S. Lewis, Nature414, 589 (2001) . 5. C. Perkins, A. W. Weimar, Int. J. Hydrogen Energy (in press). 6. J. Alper, Science 299, 1686 (2003) [MEDLINE]; F. Gloaguen et al., Inorg. Chem. 41, 6573 (2002) [MEDLINE]; J.−Z. Wu et al., Inorg. Chem. 43, 5795 (2004) [MEDLINE]; K. N. Ferreira et al., Science 303, 1831 (2004) [MEDLINE]. 7. L. Schlapbach, A. Zuttel, Nature 414, 353 (2001) [INSPEC]; W. Grochala, P. P. Edwards, Chem. Rev. 104, 1283 (2004) [MEDLINE]. 8. N. L. Rosi et al., Science 300, 1127 (2003) [INSPEC]. 9. P. Hoffmann, Tomorrow's Energy: Hydrogen, Fuel Cells, and the Prospects for a Cleaner Planet, MIT Press, Cambridge, MA (2001). 10. J. Larminie, A. Dicks, Fuel Cell Systems Explained, 2nd ed., Wiley, Hoboken, NJ (2003). 11. N. M. Markovic, P. J. Ross, Surf. Sci. Rep. 45, 117 (2002) . 12. P. L. Gai, Top. Catal. 21, 161 (2002) [CAS]. 13. A. Mattsson, Science 298, 759 (2002) [MEDLINE]. 14. Q. Li, R. He, J. O. Jensen, N. J. Bjerrum, Chem. Mater. 15, 4896 (2003) [CAS]. 15. B. Freese, Coal: A Human History, Perseus, Cambridge, MA (2003). 15. B. Freese, Coal: A Human History, Perseus, Cambridge, MA (2003).
Source: http://scitation.aip.org/journals/doc/PHTOAD-ft/vol_57/iss_12/39_1.shtml
1 2 3 4 5 6 7 8 9 10 Newest articles
|
|
Sub quantum space and interactions from photon to fermions and bosons |
Interesting articles
Since 1962 I doubted on Newton's laws. I did not accept the infinitive speed and I found un-vivid the laws of gravity and time.
I learned the Einstein's Relativity, thus I found some answers for my questions. But, I had another doubt of Infinitive Mass-Energy. And I wanted to know why light has stable speed?