Another peculiarly quantum property is that the wave-like
nature of particles leads to interference effects that violate our
usual notions of how probability works. Two
processes, which when described in a particle language seem quite
distinct, actually represent two different contributions to an overall
probability amplitude.
The rule for probability in quantum mechanics is that probability
is the square of the absolute value of the relevant probability
amplitude.
Two processes that can be distinguished by measurement have
separate probabilities, and these probabilities add in the usual way.
The peculiarity comes about when the processes are not experimentally
distinguishable, despite their different particle-language
descriptions.
An Example of Particle Interference
For example, consider the following
Feynman diagrams.
Both represent a process where an electron and a positron collide and
an electron and a positron emerge traveling in different directions
(what we call a scattering process).
We can read these diagrams as if they are a pictorial
representation of a particle process that starts at the left and ends
at the right of the picture.
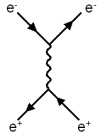 |
In the first diagram, the electron emits a photon and the
positron absorbs it, thus changing the direction of both. |
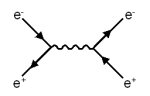 |
In the second diagram, the electron and positron meet and
annihilate, or disappear. For a short time there is only a single
virtual
photon present, which then disappears to produce a new electron
and positron pair, traveling apart in different directions from
the initial pair. |
These two pictures, and the words we use to "describe" what they
represent, certainly appear to be two very different processes. But,
we do not observe the intermediate stages and cannot do so without
changing the outcome of the experiment.
The Mathematics of Interference Calculations
Feynmans prescription assigns a complex number to each diagram, let
us write these as A and B. (The values of A and B depend on the
momenta and energy of the particles.) The probability of a given
scattering occurring is given by |A+B|2.
There is no way to say which of the two underlying processes
represented by the two diagrams actually occurred. Furthermore, we
cannot even say there is a probability of each process and then add
the probabilities, since |A+B|2 is not the same number as
|A|2 + |B|2 .
For example, let consider A= 5 and B = -3. Then we might think the
probability of the process represented by A was |A|2= 25,
while that represented by |B|2= 9. Given this, we would be
tempted to assign the probability 34 to having either the A or the B
process occur -- but the quantum answer is |A+B|2 = 22=
4. That is, the two processes interfere with one another and both
contribute to make the net result smaller than it would be if either
one alone were the only way to achieve the process!
This is the nature of quantum theories -- unobserved intermediate
stages of a process cannot be treated by the ordinary rules of
everyday experience.
In both diagrams, the photons that appear at intermediate stages
are
virtual particles
that are not observable.
Quantum Mechanics
Quantum mechanics is the description of physics at the scale of
atoms, and the even smaller scales of
fundamental
particles.
Quantum theory is the language of all particle theories. It is
formulated in a well-defined mathematical language. It makes
predictions for the relative probabilities of the various possible
outcomes, but not for which outcome will occur in any given
case. Interpretation of the calculations, in words and images, often
leads to statements that seem to defy common sense -- because our
common sense is based on experience at scales insensitive to these
types of quantum peculiarities.
Because we do not directly experience objects on this scale, many
aspects of quantum behavior seem strange and even paradoxical to us.
Physicists worked hard to find alternative theories that could remove
these peculiarities, but to no avail.
The word quantum means a definite but small amount. The basic
quantum constant h, known as
Planck's constant,
is 6.626069 x 10-34 Joule seconds.
Because the particles in high-energy experiments travel at close to
the speed of light, particle theories are
relativistic
quantum field theories.
Lets look at just a few, of the many, quantum concepts that will be
stated without explanation.
In quantum theories, energy and momentum have a definite
relationship to wavelength. All particles have properties that are
wave-like (such as interference) and other properties that are
particle-like (such as localization). Whether the properties are
primarily those of particles or those of waves, depends on how you
observe them.
For example, photons are the quantum particles associated with
electromagnetic waves.
For any frequency, f, the photons each carry a definite
amount of energy (E = hf).
Only by assuming a particle nature for light with this relationship
between frequency and particle energy could Einstein explain the
photoelectric effect.
Conversely,
electrons
can behave like waves and develop interference patterns.
| |
Discrete Energy, Momenta, and Angular Momenta
|
In classical physics, quantities such as energy and angular
momentum are assumed to be able to have any value. In quantum physics
there is a certain discrete (particle-like) nature to these
quantities.
For example, the angular momentum of any system can only come in
integer multiples of h/2, where h is Planck's Constant.
Values such as (n+1/4)h are simply not allowed.
Likewise, if we ask how much energy a beam of light of a certain
frequency, f, deposits on an absorbing surface during any
time interval, we find the answer can only be nhf, where
n is some integer. Values such as (n+1/2)hf are
not allowed.
To get some idea of how counter-intuitive this idea of discrete
values is, imagine if someone told you that water could have only
integer temperatures as you boiled it. For example, the water could
have temperatures of 85º, 86º or 87º, but not 85.7º or 86.5º. It would
be a pretty strange world you were living in if that were true.
The world of quantum mechanics is pretty strange when you try to
use words to describe it.
| |
States and Quantum Numbers
|
In quantum mechanics, systems are described by the set of possible
states in which they may be found. For example, the electron
orbitals familiar in chemistry are the set of possible
bound states
for an electron in an atom.
Bound states are labeled by a set of quantum numbers that
define the various conserved quantities associated with the state.
These labels are pure numbers that count familiar discrete quantities,
such as electric
charge,
as well as energy, and angular momentum, which can only have certain
discrete values bound quantum systems.
| |
Wave Function or Probability Amplitude
|
A state is described by a quantity that is called a wave-function
or probability amplitude. It is a complex-number-valued function of
position, that is a quantity whose value is a definite complex number
at any point in space. The probability of finding the particle
described by the wave function (e.g. an electron in an atom) at that
point is proportional to square of the absolute value of the
probability amplitude.
The "pictures" of orbitals in an introductory chemistry textbook
are a representation of the region within which the wave function
gives a high probability of finding an electron for that state.
We can also talk about quantum states for freely moving particles.
These are states with definite momentum, p, and energy
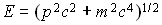 . The
associated wave has frequency given by f = pc/h where c
is the speed of light.
. The
associated wave has frequency given by f = pc/h where c
is the speed of light.
Another peculiarly quantum property is
that the wave-like nature of particles leads to interference effects
that violate our usual notions of how probability works. Two
processes, which when described in a particle language seem quite
distinct, actually represent two different contributions to an overall
probability amplitude.
The rule for probability in quantum mechanics is that probability
is the square of the absolute value of the relevant probability
amplitude.
Two processes that can be distinguished by measurement have
separate probabilities, and these probabilities add in the usual way.
The peculiarity comes about when the processes are not experimentally
distinguishable, despite their different particle-language
descriptions.
An Example of Particle Interference
For example, consider the following
Feynman diagrams.
Both represent a process where an electron and a positron collide and
an electron and a positron emerge traveling in different directions
(what we call a scattering process).
We can read these diagrams as if they are a pictorial
representation of a particle process that starts at the left and ends
at the right of the picture.
 |
In the first diagram, the electron emits a photon and the
positron absorbs it, thus changing the direction of both. |
 |
In the second diagram, the electron and positron meet and
annihilate, or disappear. For a short time there is only a single
virtual
photon present, which then disappears to produce a new electron
and positron pair, traveling apart in different directions from
the initial pair. |
These two pictures, and the words we use to "describe" what they
represent, certainly appear to be two very different processes. But,
we do not observe the intermediate stages and cannot do so without
changing the outcome of the experiment.
The Mathematics of Interference Calculations
Feynmans prescription assigns a complex number to each diagram, let
us write these as A and B. (The values of A and B depend on the
momenta and energy of the particles.) The probability of a given
scattering occurring is given by |A+B|2.
There is no way to say which of the two underlying processes
represented by the two diagrams actually occurred. Furthermore, we
cannot even say there is a probability of each process and then add
the probabilities, since |A+B|2 is not the same number as
|A|2 + |B|2 .
For example, let consider A= 5 and B = -3. Then we might think the
probability of the process represented by A was |A|2= 25,
while that represented by |B|2= 9. Given this, we would be
tempted to assign the probability 34 to having either the A or the B
process occur -- but the quantum answer is |A+B|2 = 22=
4. That is, the two processes interfere with one another and both
contribute to make the net result smaller than it would be if either
one alone were the only way to achieve the process!
This is the nature of quantum theories -- unobserved intermediate
stages of a process cannot be treated by the ordinary rules of
everyday experience.
In both diagrams, the photons that appear at intermediate stages
are
virtual particles
that are not observable.